Abstract
Background:The identification of effective therapies based on targeted interventions for human cancers faces the challenges of genetic and epigenetic heterogeneity underlying the disease. Large-scale sequencing efforts have uncovered a spectrum of mutations in many hematologic malignancies, suggesting that combinations of agents will be required to treat these diseases effectively. Combinatorial approaches will also be critical for combating the emergence of genetically heterogeneous subclones, rescue signals in the microenvironment, and tumor-intrinsic feedback pathways that all contribute to disease relapse.
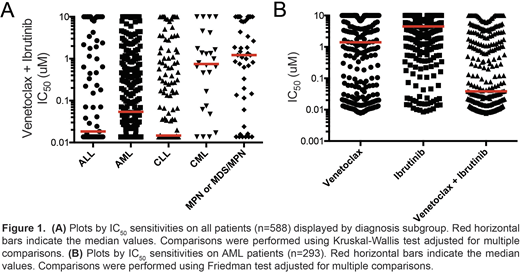
Methods:We used a functional ex vivo screening assay to identify small-molecule targeted inhibitors and inhibitor combinations demonstrating selective efficacy across broad categories of leukemia. Primary mononuclear cells isolated from leukemia patients (n=588) were plated in the presence of a panel of graded concentrations of over 120 single-agent inhibitors or combinations spanning multiple drug classes. Leukemia specimens from 5 diagnosis subgroups included acute myeloid leukemia (AML; n=293), acute lymphoblastic leukemia (ALL; n=83), chronic lymphocytic leukemia (CLL; n=140), chronic myeloid leukemia (CML; n=26) and myeloproliferative neoplasms/myelodysplastic syndrome (MPN or MDS/MPN; n=46) patients. The combinations were designed as drug pairs that target non-overlapping biological pathways and comprise drugs from different classes, preferably with Food and Drug Administration approval. IC50 and AUC values were derived from probit-based regression for each response curve. Mutational status for FLT3-ITD and NPM1 were compiled from clinical labs or by capillary electrophoresis using a QiaXcel instrument. Disease status was obtained from clinical chart review. Single and combination drug treatment IC50 and AUC values were compared within groups by Friedman test, across groups by Kruskal-Wallis test, and with continuous variables by Spearman rank correlation.
Results:Among 588 unique leukemia patients evaluated, we observed that the combination of venetoclax (a BCL2 inhibitor) with ibrutinib(a BTK inhibitor) showed enhanced benefit above that for either single agent in both myeloid and lymphoid malignancies, including AML, ALL, and CLL. Expanded analyses of combination sensitivity in AML patients showed no significant associations with age, gender, or ELN prognostic risk. Comparison of AML samples by disease treatment status indicated a slightly greater sensitivity in relapsed/refractory (n=42) versus newly diagnosed (n=115) AML samples by both AUC and IC50 (p=0.028 and 0.026, respectively). Among AML samples, FLT3-ITD and NPM1 mutation status was available for 204 and 188 patients, respectively. Venetoclax + ibrutinib was significantly more effective in patient samples harboring FLT3-ITD or NPM1 mutations by both AUC (p=0.0002 and p=0.0032, respectively) and IC50 (p=0.0002 and p=0.021, respectively). Furthermore, among 112 AML samples with RNAseq data available, sensitivity to the combination (AUC) was significantly correlated with gene expression for BCL2 and BTK (Spearman r: -0.48 and -0.21, respectively). Evaluation of the combination using an expanded 7x7 concentration matrix on AML cell lines revealed synergy for the majority of drug-pair concentrations by the Bliss independence model. Analysis to align additional clinical and genetic features for leukemia patient samples with drug sensitivities is in progress and results with be presented at the meeting.
Conclusion:Both myeloid and lymphoid-derived primary leukemia cells show sensitivity to combined inhibition of BCL2 and BTK with the combination of venetoclax and ibrutinib, suggesting this drug pair may have broad therapeutic indications.
Tyner:Vivid Biosciences: Membership on an entity's Board of Directors or advisory committees; Takeda: Research Funding; Genentech: Research Funding; Gilead: Research Funding; Constellation: Research Funding; Janssen: Research Funding; AstraZeneca: Research Funding; Incyte: Research Funding; Array: Research Funding; Aptose: Research Funding. Danilov:TG Therapeutics: Consultancy; Takeda Oncology: Research Funding; Aptose Biosciences: Research Funding; Genentech: Consultancy, Research Funding; Astra Zeneca: Consultancy; Gilead Sciences: Consultancy, Research Funding; Bayer Oncology: Consultancy, Research Funding; Verastem: Consultancy, Research Funding. Druker:Aileron Therapeutics: Consultancy; Third Coast Therapeutics: Membership on an entity's Board of Directors or advisory committees; Aptose Therapeutics: Consultancy, Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Patient True Talk: Consultancy; Vivid Biosciences: Membership on an entity's Board of Directors or advisory committees; GRAIL: Consultancy, Membership on an entity's Board of Directors or advisory committees; ARIAD: Research Funding; Oregon Health & Science University: Patents & Royalties; Fred Hutchinson Cancer Research Center: Research Funding; Cepheid: Consultancy, Membership on an entity's Board of Directors or advisory committees; Millipore: Patents & Royalties; Leukemia & Lymphoma Society: Membership on an entity's Board of Directors or advisory committees, Research Funding; Gilead Sciences: Consultancy, Membership on an entity's Board of Directors or advisory committees; McGraw Hill: Patents & Royalties; Henry Stewart Talks: Patents & Royalties; Novartis Pharmaceuticals: Research Funding; Monojul: Consultancy; ALLCRON: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy; Beta Cat: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees; Bristol-Meyers Squibb: Research Funding; MolecularMD: Consultancy, Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Blueprint Medicines: Consultancy, Equity Ownership, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.